COVID-19 Scientific and Public Health Policy Update – (2 March 2022)
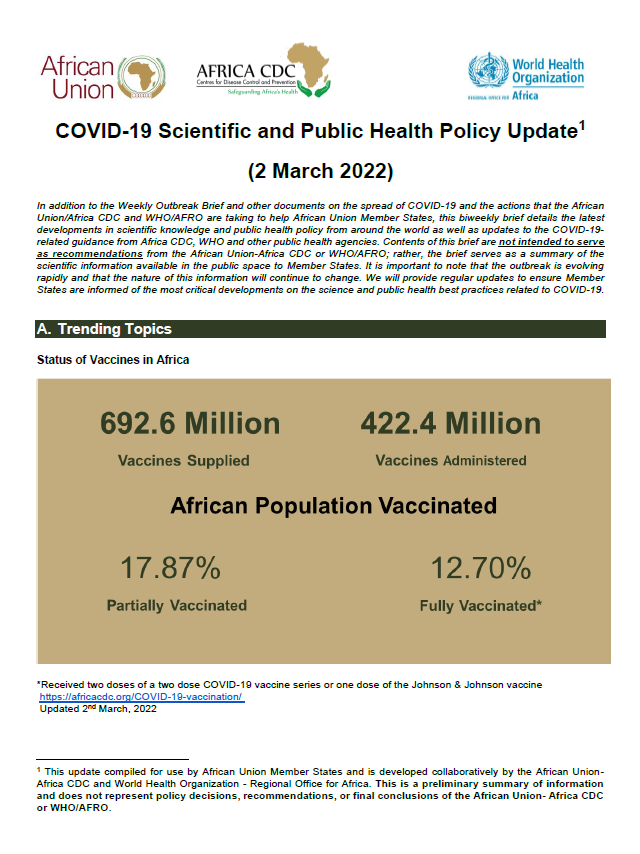
In addition to the Weekly Outbreak Brief and other documents on the spread of COVID-19 and the actions that the African Union/Africa CDC and WHO/AFRO are taking to help African Union Member States, this biweekly brief details the latest developments in scientific knowledge and public health policy from around the world as well as updates […]
Partnerships for African Vaccine Manufacturing (PAVM) Framework for Action

Foreword It gives me great pleasure to present the Framework for Action (FFA) that the Partnerships for African Vaccine Manufacturing (PAVM) has prepared under the supervision of the African Centers for Disease Control and Prevention (Africa CDC). The Framework for Action sets forth the key diagnostic findings on the current vaccine manufacturing environment in Africa […]
Outbreak Brief 111: Coronavirus Disease 2019 (COVID-19) Pandemic

Outbreak Update: As of 28 February 2022, a global total of 434,112,380 COVID-19 cases and 5,944,734 related deaths (case fatality ratio (CFR): 1.4%) have been reported by 227 countries and territories to the World Health Organization (WHO). The distribution of cumulative cases (proportion of global cases) from the WHO regions (excluding Africa) are as follows: […]
Report on the Virtual joint Meeting of African Ministers of Health, ICT and Transport

Introduction The COVID-19 pandemic in Africa continues to occur in waves with some countries already experiencing third and fourth waves. Whilst a recovery of sorts is underway, it requires concerted regional efforts to be sustained. The continued socioeconomic impacts of the pandemic vindicate the decision by the African Union Commission through the Africa CDC to […]
COVID-19 Scientific and Public Health Policy Update – (16 February 2022)
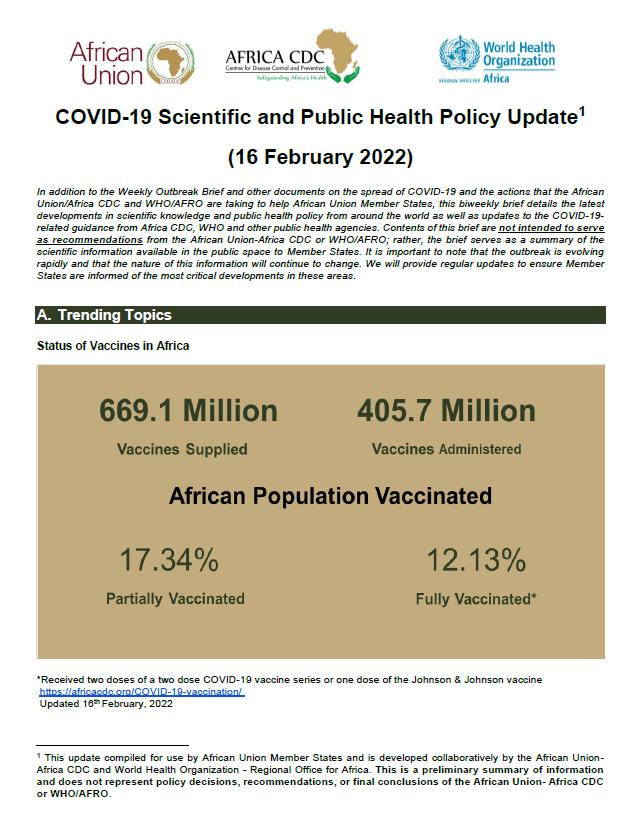
In addition to the Weekly Outbreak Brief and other documents on the spread of COVID-19 and the actions that the African Union/Africa CDC and WHO/AFRO are taking to help African Union Member States, this biweekly brief details the latest developments in scientific knowledge and public health policy from around the world as well as updates […]
PUBLIC HEALTH GRAND ROUNDS: A New Public Health Order in the 21st Century

Omicron and other COVID-19 variants of concern – where are we now? All viruses, including SARS-CoV-2 mutate over time naturally. Many of these changes do not have much impact on the virus’ properties. However, with time and due to various contributing factors, some changes may result in altered characteristics of the virus including its ability […]
Guidance on administration of COVID-19 vaccine boosters in Africa

Context As part of the “Strategy to Achieve Global COVID-19 Vaccination by mid-2022”, global targets of 40% population coverage by end of 2021 and 70% coverage by June 2022 have been set by the World Health Organization (WHO), to successfully prevent severe illness and deaths, minimize social disruption and economic consequences of COVID-19, curtail the […]
Outbreak Brief 109: Coronavirus Disease 2019 (COVID-19) Pandemic

Outbreak Update: As of 14 February 2022, a global total of 410,522,545 COVID-19 cases and 5,811,307 related deaths (case fatality ratio (CFR): 1.4%) have been reported by 227 countries and territories to the World Health Organization (WHO). The distribution of cumulative cases (proportion of global cases) from the WHO reporting regions (excluding Africa) are as […]
Outbreak Brief 108: Coronavirus Disease 2019 (COVID-19) Pandemic

Outbreak Update: As of 7 February 2022, a global total of 394,334,858 COVID-19 cases and 5,735,684 related deaths (case fatality ratio (CFR): 1.4%) have been reported by 227 countries and territories to the World Health Organization (WHO). The distribution of cumulative cases (proportion of global cases) from the WHO reporting regions (excluding Africa) are as […]
Outbreak Brief 107: Coronavirus Disease 2019 (COVID-19) Pandemic

Outbreak Update: As of 31 January 2022, a global total of 373,180,340 COVID-19 cases and 5,659,292 related deaths (case fatality ratio (CFR): 1.5%) have been reported by 227 countries and territories to the World Health Organization (WHO). The distribution of cumulative cases (proportion of global cases) from the WHO reporting regions (excluding Africa) are as […]
