Vaccine R&D and Vaccine Manufacturing Competency Frameworks

FOREWORD I am delighted to introduce the Competency Frameworks for Vaccine Manufacturing and Research and Development Competency Frameworks, which have been prepared through collaborative efforts with key stakeholders in the industry, supported by Africa Centres for Disease Control and Prevention (Africa CDC). The Competency Frameworks are essential tools for ongoing assessment of training needs, the […]
Current and planned vaccine manufacturing in Africa: Results from a joint assessment by Africa CDC, CHAI, and PATH

Study methodology Between December 2022 and March 2023, a collaborative team with representatives from Africa CDC, CHAI, and PATH engaged vaccine manufacturers across Africa on their current and planned production Current and planned vaccine manufacturing in Africa Results from a joint assessment by Africa CDC, CHAI, and PATH capacity, technical and commercial capabilities, and supporting […]
Africa Region Subject Matter Expert (Af-RSME) under the Regional Training and Certification Program for Biosafety and Biosecurity Professionals in the Africa Region

Introduction The Africa Centres for Disease Control and Prevention (Africa CDC) Biosafety and Biosecurity Initiative was launched by the Africa CDC in April 2019 with the aim of strengthening the African Union (AU) Member States’ biosafety and biosecurity systems and enabling them to comply with national and international requirements for biosafety and biosecurity including the […]
COVID-19 Scientific and Public Health Policy Update – (8 June 2022)
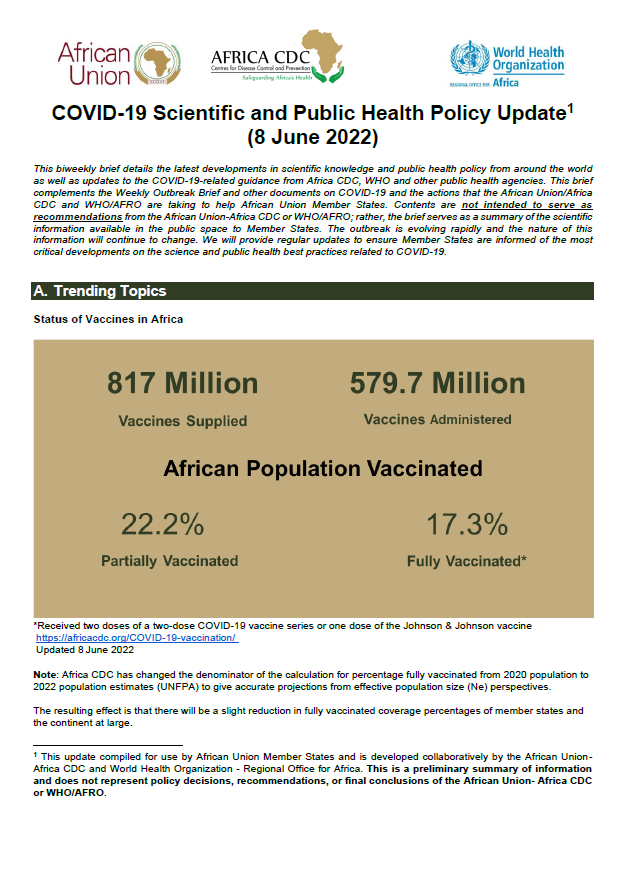
This biweekly brief details the latest developments in scientific knowledge and public health policy from around the world as well as updates to the COVID-19-related guidance from Africa CDC, WHO and other public health agencies. This brief complements the Weekly Outbreak Brief and other documents on COVID-19 and the actions that the African Union/Africa CDC […]
COVID-19 Scientific and Public Health Policy Update – (27 April 2022)

This biweekly brief details the latest developments in scientific knowledge and public health policy from around the world as well as updates to the COVID-19-related guidance from Africa CDC, WHO and other public health agencies. This brief complements the Weekly Outbreak Brief and other documents on COVID-19 and the actions that the African Union/Africa CDC […]
Viruses, vaccines and variants: What can we expect next?
Partnership for African Vaccine Manufacturing (PAVM) From Aspiration To Action

1- CONTEXT12-13 April 2021 African Vaccine Manufacturing SummitThe African Union Commission (AUC) and Africa Centres for Disease Control (Africa CDC) hosted a two-day high-level Summit under the theme “Expanding Africa’s Vaccine Manufacturing for Health Security: Building back better, bolder and bigger”. Amongst the continental leadership in attendance were:• H.E Moussa Faki Mahamat, Chairperson, African Union […]
Africa CDC – Moderna Scientific Exchange

Join the meeting: https://zoom.us/j/3337716453?pwd=NjAremxxQjJRRXVHaGt0VUNzd3lZdz09
Podcast: Building clinical trial capacity for COVID-19 and other vaccines in Africa

[Africa CDC Podcast series: Courtesy Prof. Rosanna Peeling, London School of Hygiene and Tropical Medicine and Director of the International Diagnostic Centre – Prof Salim Abdool Karim, Director of CAPRISA and AFTCOR Steering Committee Member, provides some insights on ‘Building clinical trial capacity for COVID-19 and other vaccines in Africa’.]
Guidance on Diagnosis and Management of People with Post-Acute COVID-19 Syndrome

BACKGROUND This guidance covers diagnosis and care of patients with long-term effects of COVID-19. It makes recommendations for the care of adults and children who have new or ongoing symptoms 4 weeks or more after the start of acute COVID-19. It is meant for health and care practitioners. This interim document has been developed by […]
