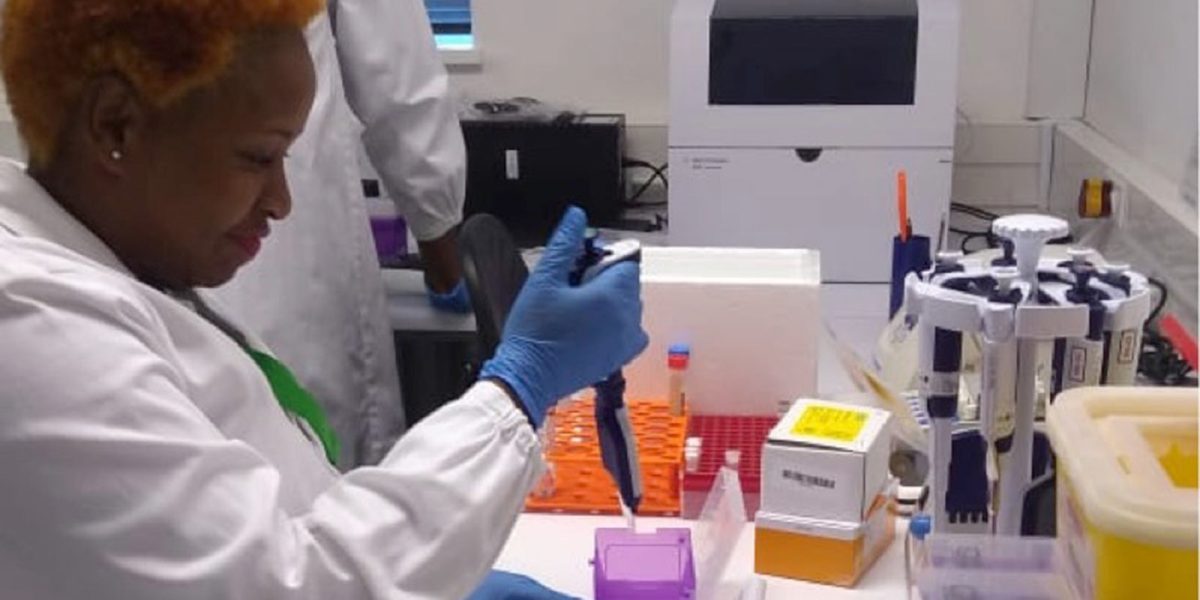
Improved laboratory diagnostic systems and networks are critical in supporting national and regional disease surveillance systems and outbreak responses, and in ensuring accuracy of diagnosis for effective case management in clinical care and public health emergency responses. Quality management is essential to achieve the highest level of accuracy and reliability of laboratory tests results. However, most medical laboratories in Africa are not accredited, and veterinary diagnostic and environmental laboratories are not being supported to improve their quality system.
Africa CDC, through the Regional Collaborating Centres, is working with different partners to advance laboratory quality management system in Africa through:
- Provision of a systematic process-oriented approach to meeting quality objectives in human, animal, environmental and food analysis laboratories.
- Support for consistent, high-quality, cost-effective and efficient laboratory services with implementation of quality management systems in Member States.
- Support for the development, implementation and maintenance of relevant policies, processes and procedures for quality system essentials.
- Training of a pool of trainers who will help cascade laboratory quality management trainings in each Member State to strengthen diagnostic, detection and investigation systems.
- Support for networking among professionals across different sectors for immediate detection and response to public health threats.
- Sensitization of selected laboratory professionals on quality management systems.
- Provision of basic knowledge and understanding of the ISO 15189:2012 and audit skills.
- Support for laboratory professional to conduct audits using the SLIPTA tool and provision of corrective actions to improve the quality system of public health laboratories.