Africa’s Leadership Role in the Development and Access to Potential COVID-19 Vaccine: Rapporteur’s Report for Day Two

SPECIAL SESSION II: Role of the Private Sector in COVID 19 Vaccines Development and Access Needs for Private Sector Engagement: Pooling the research: public and private resources needed to fund research and development process not only for COVID 19. With 25% of vaccine consumption in Africa, barriers to investments in the business of vaccine need […]
Africa’s Leadership Role in the Development and Access to Potential COVID-19 Vaccine: Highlights for Day Two

SPECIAL SESSION II: ROLE OF THE PRIVATE SECTOR IN COVID-19 VACCINES DEVELOPMENT AND ACCESS The second day of this virtual conference started with a special session featuring six presentations on the role of the private sector in COVID-19 vaccine development and access. Dr Vera Songwe, United Nations Under-Secretary General and Executive Secretary, United Nations Economic […]
Africa’s Leadership Role in the Development and Access to Potential COVID-19 Vaccine: Highlights for Day One

SESSION 1: OPENING The meeting brought together African leaders, public health professionals, policymakers, the media, civil society, community leaders, private sector representatives, pharmaceutical industry experts, and partners to discuss a roadmap for the development of safe, efficacious, affordable, equitable and accessible COVID-19 vaccine in Africa, with the involvement of Africans. H.E. President Cyril Ramaphosa, Chairperson […]
AFRICA’S LEADERSHIP ROLE IN COVID-19 VACCINE DEVELOPMENT AND ACCESS

VIRTUAL CONFERENCE | 24-25 JUNE 2020 Recognizing the urgent need for collaboration, cooperation, and coordination to ensure that Africa plays a leadership role in the development and access to potential vaccines, this two-day virtual conference, under the leadership of H.E. Moussa Faki Mahamat, Chairperson of the African Union Commission (AUC), will bring together healthcare professionals, […]
Interim guidance on the use of rapid antibody tests for COVID-19 response

The purpose of this document is to provide guidance for Ministries of Health (MOHs) in the African Union Member States in the selection and application of rapid antibody tests to respond to the COVID-19 pandemic. This interim guidance will serve as a reference for the national laboratory leads and experts when selecting and prioritizing laboratory […]
Partnership to Accelerate COVID-19 Testing (PACT) in Africa – Resources

To help increase continental testing efforts and reduce COVID-19 transmission in Africa, Africa CDC has launched the Partnership to Accelerate COVID-19 Testing (PACT): Test, Trace, Treat. PACT will mobilize experts, community workers, supplies and other resources to TEST, TRACE and TREAT COVID-19 cases in a timely manner to minimize the impact of the pandemic on […]
Africa CDC Director’s message on the rollout of PACT

Support Partnership to Accelerate COVID-19 Testing (PACT) for timely testing, tracing and treatment to reduce transmission of COVID-19 in Africa
Roll Out : Partnership to Accelerate COVID-19 Testing (PACT) in Africa

The Africa Centres for Disease Control and Prevention (Africa CDC) is pleased to invite you to the roll out of the Partnership to Accelerate COVID-19 Testing (PACT) in Africa. PACT is a continental strategy to help Member States limit COVID-19 transmission by ensuring uninterrupted supply of diagnostics and medical supplies, as well as frontline personnel, needed […]
Africa CDC COVID-19 Response Update, 31 May 2020
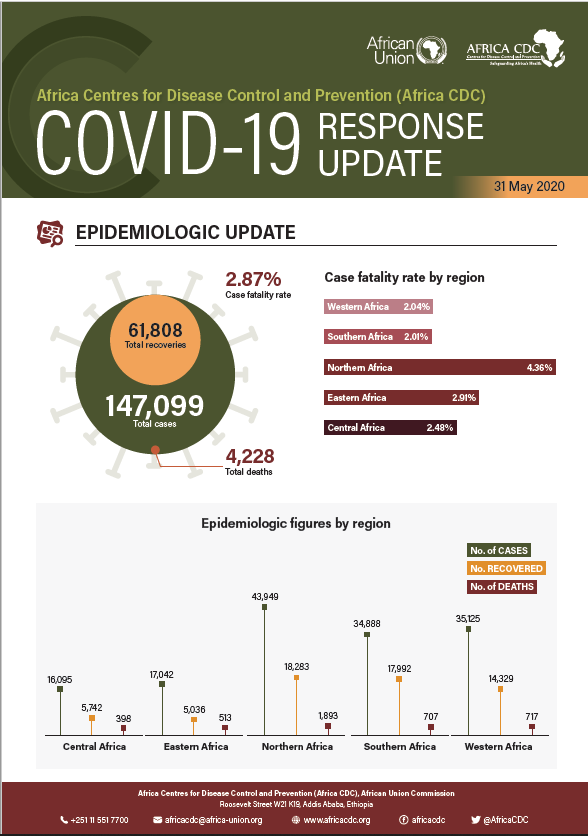
Graphic illustration of where we were with COVID-19 response as of 31 May 2020. 1- EPIDEMIOLOGIC UPDATE 2- EQUIPMENT SUPPORT TO MEMBER STATES 3- NUMBER OF HEALTHCARE WORKERS TRAINED 4- STRATEGIC ACTION UPDATE 5- PERSONNEL DEPLOYMENTS BY COUNTRY 6- AFRICA CDC TARGETS 7- COVID-19 tests conducted by Member States as of 31 July 2020
Webinar: Manufactures overview on COVID 19 Molecular diagnostics tools, ABBOT and BIO RAD company (EN)
Topic: Manufactures overview on COVID 19 Molecular diagnostics tools, ABBOT and BIO RAD company Description: Overview on available COVID 19 laboratory test kits, what genes are targeted, status regarding regulatory approval performance, run time and other technical consideration. Presenter: MS Danijela Lucic ( senior Scientific Affairs Manager, Gobal infectious disease, Abbot). Dr Marcus Neusser (EMEA […]
