Consultative Workshop on Revised COVID-19 Testing Strategy
COVID-19 Test to Treat Guidelines for African Union Member States

EXECUTIVE SUMMARY In response to the outbreak, the Africa Centres for Disease Control and Prevention (Africa CDC) has been supporting African Union Member States in responding to the COVID-19 pandemic through a variety of interventions such as non-pharmaceutical interventions, quarantine, testing, isolation, contact tracing, and clinical management. The Test to Treat guideline aims to increase […]
African Union List of Certified Assessors for the Regulatory and Certification Framework for Institutions Handling High Risk Pathogens

The Africa Centers for Disease Control (Africa CDC) launched the Biosafety and Security Initiative in April 2019 to strengthen the biosecurity and biosafety systems of African Union Member States to enable them to comply with international standards for Biosafety and Biosecurity. Recent events of public health concern and emergency of infectious diseases including the Ebola […]
The regulatory and certification framework for institutions handling high risk pathogens in the Africa Region

The World Health Organization (WHO) Joint External Evaluation (JEE) technical assessments conducted between 2016-2019 and the Global Health Security Index (GHSI) of 2021 highlighted Africa Union (AU) Member States limited capacities in Biosafety and Biosecurity. In the GHSI report none of the participating AU Member states scored above 50% in Biosecurity and only 2 with […]
Revised COVID-19 Testing Strategy, Second Edition, August, 2022

EXECUTIVE SUMMARY Testing to identify people infected with SARS-COV-2, the virus that causes COVID-19, has been central to the control of the disease. The African Union Commission, through the Africa Centres for Disease Control and Prevention (Africa CDC) and partners, implemented a number of key initiatives to expand COVID-19 testing in Africa including developing guidance […]
COVID-19 Scientific and Public Health Policy Update – (8 June 2022)
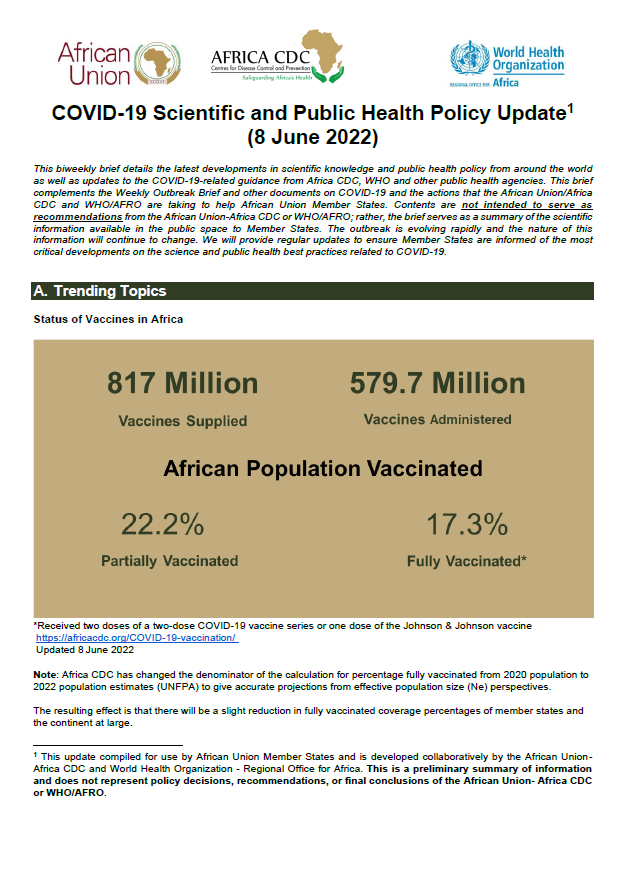
This biweekly brief details the latest developments in scientific knowledge and public health policy from around the world as well as updates to the COVID-19-related guidance from Africa CDC, WHO and other public health agencies. This brief complements the Weekly Outbreak Brief and other documents on COVID-19 and the actions that the African Union/Africa CDC […]
Interim guidance on COVID-19 Rapid Antigen self-testing to African Union Member States

EXECUTIVE SUMMARY Since the first case of COVID-19 was reported in Africa in February 2020, testing to identify individuals infected with SARS-COV-2, the virus that causes COVID-19, has been a cornerstone in controlling the spread of the disease. However, by the end of January 2022, over 94 million tests had been performed across all 55 […]
Africa PGI Webinar Series on Pathogen Genomic Surveillance in Africa

You are invited to the #Africa#PGI Webinar Series on Pathogen Genomic Surveillance in Africa.
Partnership for African Vaccine Manufacturing (PAVM) From Aspiration To Action

1- CONTEXT12-13 April 2021 African Vaccine Manufacturing SummitThe African Union Commission (AUC) and Africa Centres for Disease Control (Africa CDC) hosted a two-day high-level Summit under the theme “Expanding Africa’s Vaccine Manufacturing for Health Security: Building back better, bolder and bigger”. Amongst the continental leadership in attendance were:• H.E Moussa Faki Mahamat, Chairperson, African Union […]
Africa CDC – Moderna Scientific Exchange

Join the meeting: https://zoom.us/j/3337716453?pwd=NjAremxxQjJRRXVHaGt0VUNzd3lZdz09
