Mpox Molecular Diagnostic Tests (RT-PCR)

On 13 August 2024, Africa Centres for Disease Control and Prevention (Africa CDC) declared the ongoing mpox outbreak a Public Health Emergency of Continental Security (PHECS). The World Health Organization (WHO) followed this the next day, which extended the alert internationally as a Public Health Emergency of International Concern (PHEIC). After these declarations, many countries […]
Africa CDC Epidemic Intelligence Weekly Report, February 2025
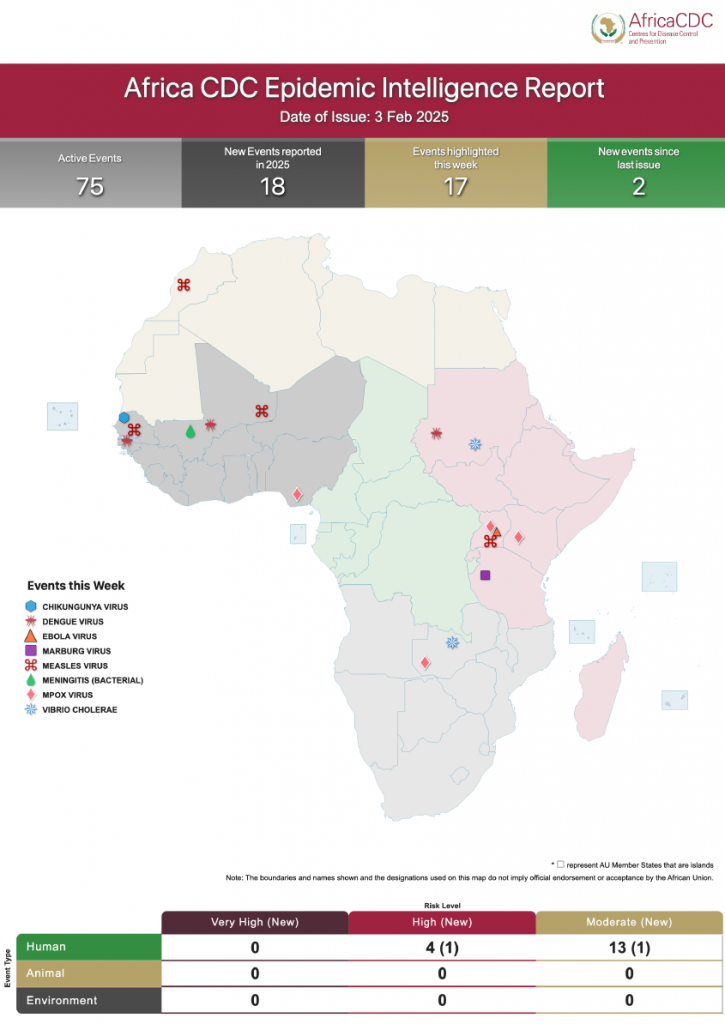
Welcome to our Epidemic Intelligence Weekly Report, your trusted source for real-time updates on priority events of concern on the continent. The narrative brought to you focuses on three main areas: the epidemiological situation, event assessment (risk assessment and geoscope) and public health interventions by the affected Member State, partners, and Africa CDC. Every week […]
Africa CDC Epidemic Intelligence Weekly Report, January 2025
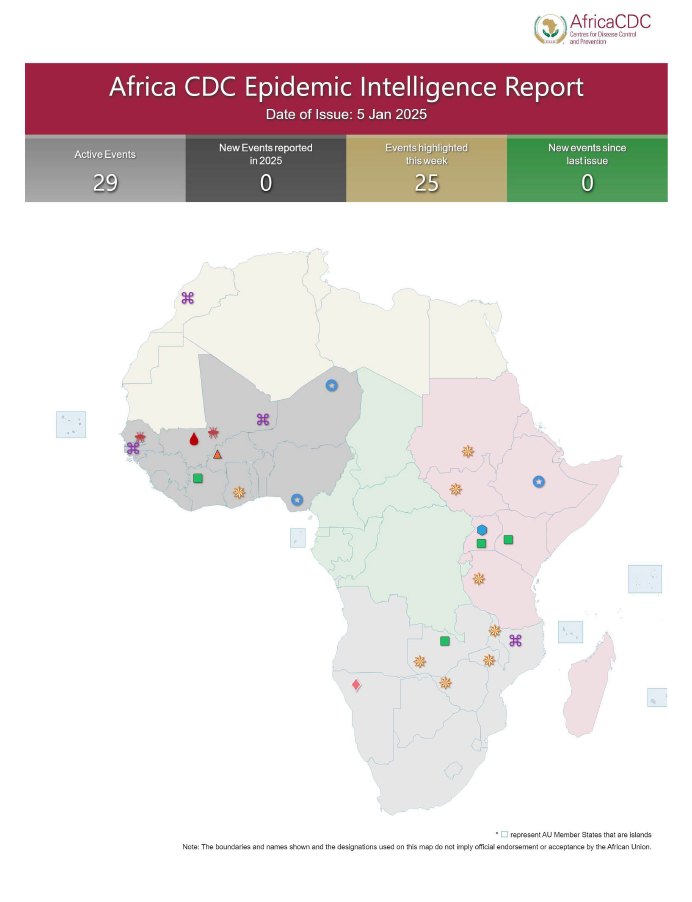
Welcome to our Epidemic Intelligence Weekly Report, your trusted source for real-time updates on priority events of concern on the continent. The narrative brought to you focuses on three main areas: the epidemiological situation, event assessment (risk assessment and geoscope) and public health interventions by the affected Member State, partners, and Africa CDC. Every week […]
Mpox Testing Strategy, November 2024

1. BACKGROUND AND RATIONALE Mpox is an emerging zoonotic disease caused by the mpox virus, a member of the Orthopoxvirus genus closely related to the variola virus that causes smallpox. Mpox was first discovered in 1958 when outbreaks of a pox-like disease occurred in monkeys kept for research. The first human case was recorded in […]
Mpox Molecular Diagnostic Tests(RT-PCR)

On 13 August 2024, Africa Centres for Disease Control and Prevention (Africa CDC) declared the ongoing mpox outbreak a Public Health Emergency of Continental Security (PHECS). The World Health Organization (WHO) followed this the next day, which extended the alert internationally as a Public Health Emergency of International Concern (PHEIC). After these declarations, many countries […]
Statement on Antigen Rapid Tests for Mpox Diagnosis, 30 October 2024

No Antigen Rapid Diagnostic Test (RDT) has demonstrated the minimum requirement for mpox testing. On 13 August 2024, Africa Centres for Disease Control and Prevention (Africa CDC) declared the ongoing mpox outbreak a Public Health Emergency of Continental Security (PHECS). This was followed the next day by the World Health Organization (WHO), which extended the […]
African Vaccine Manufacturing Mapping – Supply and Demand Landscape

This slide deck, presented at the Developing Countries Vaccine Manufacturers Network International Annual General Meeting in October 2024, describes updated results from a study to assess the current and planned state of vaccine manufacturing in Africa and provides insights into what’s needed to develop a robust and sustainable vaccine manufacturing ecosystem. We surveyed African vaccine […]
Mpox Joint Africa CDC – WHO SITREP

Executive Summary Summary of major developments during the reporting period (e.g., significant outbreaks, policy changes, vaccination campaigns, etc.). • Africa CDC and WHO established a Technical Review Committee (TRC) as an access and allocation mechanism for mpox vaccines, treatments, and diagnostics. • Nigeria was the first country to receive 10,000 doses of the JYNNEOS mpox […]
Mpox Molecular Diagnostic Tests(RT-PCR)

On 13 August 2024, Africa Centres for Disease Control and Prevention (Africa CDC) declared the ongoing mpox outbreak a Public Health Emergency of Continental Security (PHECS). The World Health Organization (WHO) followed this the next day, which extended the alert internationally as a Public Health Emergency of International Concern (PHEIC). After these declarations, many countries […]
