Ebola Outbreak in the DRC Declared Over

Kinshasa, 1 December 2025 – The Ebola outbreak in the Democratic Republic of Congo (DRC) has been declared over after 42 days without any new cases and following the recovery of the last confirmed patient. “This success is the result of exceptional determination and exemplary coordination,” said Dr Jean Kaseya, Director General of the Africa […]
Ethiopia Launches Fourth National Plan to Combat Antimicrobial Resistance

Addis Ababa, 1 December 2025 – Ethiopia has unveiled its fourth National Action Plan on Antimicrobial Resistance (AMR), reaffirming its commitment to tackling this pressing global health threat. The launch places Ethiopia among a growing number of African countries with firm strategies to address AMR, which occurs when bacteria, viruses, fungi, and parasites evolve to […]
Africa CDC dévoile une nouvelle vision pour la Sécurité et la Souveraineté Sanitaire du Continent
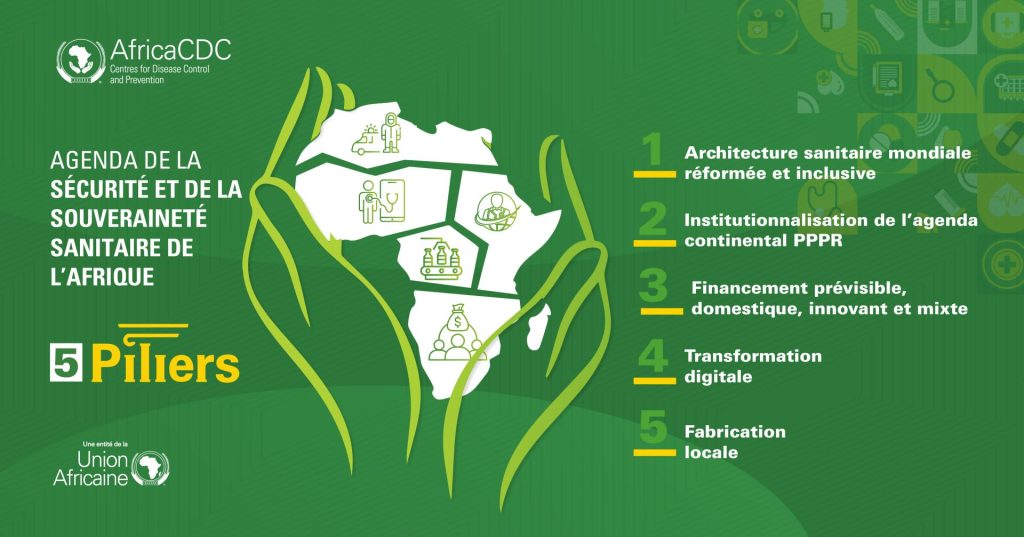
Addis-Abeba, le 21 Novembre 2025 — Les Centres africains de contrôle et de prévention des maladies (Africa CDC) ont dévoilé aujourd’hui une vision renouvelée de l’Agenda pour la Sécurité et la Souveraineté Sanitaire de l’Afrique (AHSS), destiné à protéger le continent face à la montée des menaces sanitaires tout en réduisant la dépendance aux systèmes […]
Africa CDC Launches AGARI, a Continent-Wide Genomic Data Platform to Strengthen Outbreak Response

Addis Ababa, 21 November 2025 — The Africa Centres for Disease Control and Prevention (Africa CDC) has launched an online platform that will allow researchers across the continent to share vital genomic data on disease-causing pathogens of concern to Africa. Known as the Africa Genome Archiving for Response and Insight (AGARI), the platform is the product […]
Africa CDC Unveils a New Vision for Health Security and Sovereignty Across the Continent
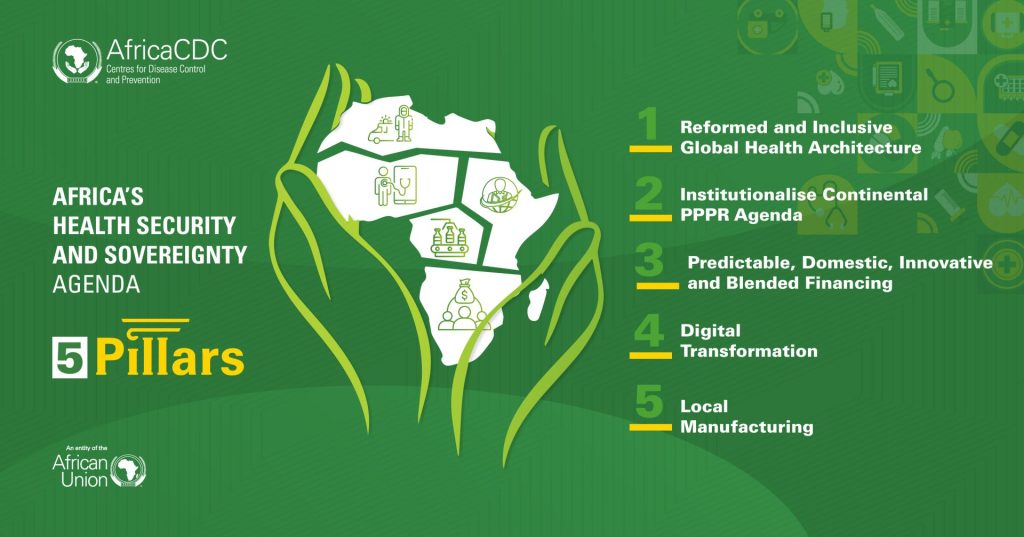
Addis Ababa, 21 November 2025 — The Africa Centres for Disease Control and Prevention (Africa CDC) today unveiled a renewed vision for Africa’s Health Security and Sovereignty (AHSS) Agenda to safeguard the continent against rising health threats while reducing dependency on external systems, manufacturing, procurement, supply chains, and financing. The AHSS Agenda builds on the foundations […]
Africa CDC Welcomes Pandemic Fund Selections and Strengthened Support for African Preparedness

Addis Ababa, 20 November 2025 — The Africa Centres for Disease Control and Prevention (Africa CDC) participated in the Pandemic Fund (PF) Board Meeting held in Kigali, Rwanda, from 17–19 November 2025. The Pandemic Fund remains the world’s leading financing mechanism dedicated to strengthening Pandemic Prevention, Preparedness, and Response (PPPR), with around 50% of its global […]
Africa CDC Statement on Confirmed Marburg Virus Disease in Jinka, Southern Region, Ethiopia

Addis Ababa, Ethiopia, 15 November 2025 — The Africa Centres for Disease Control and Prevention (Africa CDC) acknowledges the confirmation by the Federal Ministry of Health of Ethiopia and the Ethiopian Public Health Institute (EPHI) of an outbreak of Marburg virus disease (MVD) in Jinka, Southern Region. As of 14 November 2025, Marburg virus disease (MVD) has been […]
Africa CDC Launches Operational Research on Malaria in Southern Africa
Lusaka, Zambia | 12 November 2025 — The Africa Centres for Disease Control and Prevention (Africa CDC) has launched operational research on malaria in Lesotho, Namibia and Zimbabwe, supported by a US$150,000 grant from the World Bank. This initiative, which follows recurring malaria outbreaks in the southern region, will be conducted from 14 November to 12 December 2025, and aims to strengthen […]
Africa CDC Statement on Suspected Viral Haemorrhagic Fever in Jinka, Southern Region, Ethiopia

13 November 2025, Addis Ababa – The Africa Centres for Disease Control and Prevention (Africa CDC) is closely monitoring reports of a suspected viral haemorrhagic fever (VHF) in Jinka, Southern Region, Ethiopia. On 12 November 2025, the Ethiopia Public Health Institute (EPHI) notified Africa CDC of eight suspected cases, with clinical samples collected and submitted […]
Africa CDC Pushes for Country-Level Action in Version 2.0 of the African Union Framework for AMR (2026–2030)

Addis Ababa, Ethiopia | 7 November 2025 — A three-day continental meeting hosted at the Africa Centres for Disease Control and Prevention (Africa CDC) headquarters closed with a strong call to translate antimicrobial resistance (AMR) strategies into country-level action, as African Union (AU) organs and Member States move to finalise a strengthened Framework 2.0 to guide implementation from 2026 to 2030. Dr […]
